Fluoroquinolones and Tendon Basics
Marco Karrer
June 22, 2023
Introduction
In the following series of articles, we will take a closer look at tendons, tendon diseases (medical term tendinopathy), and the influence of fluoroquinolone antibiotics on tendons.
However, before we can address the topic of fluoroquinolone antibiotics, we must first understand what a tendon is, how it is composed, where the blood comes from, how pain actually occurs, etc. In short: We want to understand how a tendon “ticks,” how it “lives,” what it has to “struggle” with. This knowledge is essential to find the best way to deal with fluoroquinolone-associated tendon diseases.
What is a tendon?
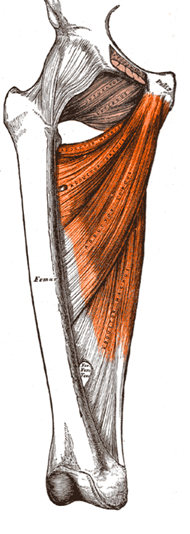
Tendon is the term used to describe the tissue that connects bone and skeletal muscle.¹ In anatomical drawings, the tendon is usually drawn as a white structure and can thus be easily distinguished from the muscles, usually drawn in red. This is illustrated in the following picture.
Attribution: colorized by Michael Gasperl, Public domain, via Wikimedia Commons
In this picture, you can see the adductor muscles drawn in red. The tendon, i.e. the white structure, connects the musculature with the femur, the thigh bone. It should not be forgotten that a tendon is always on both sides of the muscle. In the drawing above, you can’t see this very well; however, there is also a tendon that attaches the adductor muscles to the bones of the pelvis. By definition, the tendon that attaches to the mobile bone is the insertion. So in the example above, this would be the femur. Origin is where the muscle originates from the bone, which is not moved during a contraction.
What is a tendon made of?
Tendons consist predominantly of tensile collagen type I fibers arranged in the direction of muscle contraction.¹ Between the collagen fibers are some elastic fibers¹ and the actual tendon cells (tendinocytes).²
What is Collagen?
Collagen is composed of collagen molecules, which are primarily produced by fibroblasts.¹ All collagen molecules are composed of three peptide chains, which wind around each other. This means that collagen is a peptide produced by the body. Each chain consists of approximately 1000 amino acids, of which every third amino acid is glycine and in which proline and hydroxyproline are often found. However, other amino acids, such as lysine, occur too. Depending on the function and properties of the tissue, different peptide chains with different amino acid composition and sequence are used. Currently, about 40 different types of collagen are known.
The most common type in the human body is Type I collagen, found in the skin, bones, tendons, ligaments, fasciae, and intervertebral discs. Type II collagen, on the other hand, is primarily found in elastic cartilage in joints and the nucleus pulposus of intervertebral discs, and the vitreous body of the eye. Collagen type III is found primarily in the wall of blood vessels but also in lymphatic organs, the intestinal mucosa, the liver, and the surface of fat and muscle cells.¹ Because organs have different functions, collagen also needs different properties, which is achieved by a different amino acid composition and sequence. For example, collagen type I is thicker at 50-90nm than collagen type III at 20-40nm thick.
The figure below shows the formation of collagen fibrils. One can see the binding of the three peptide chains, first stored together to form procollagen. After splitting off certain areas, tropocollagen molecules are formed, incorporated into the organs in combination with other tropocollagen molecules.
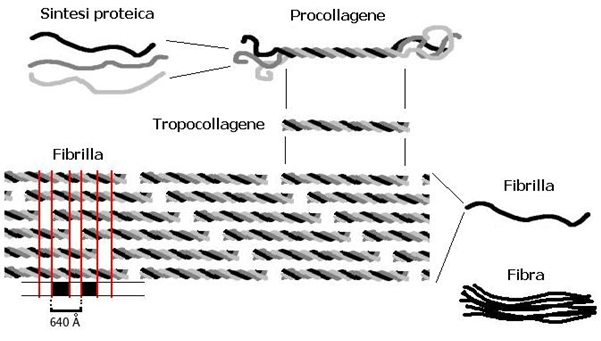
Collagen synthesis from 3 peptide chains
Attribution: User Gia.cossa edit of original work by Solitchka on fr.wikipedia, CC BY-SA
3.0 <http://creativecommons.org/licenses/by-sa/3.0/>, via Wikimedia Commons
Although collagen fibers are flexible, they are also very tear and tensile resistant and hardly stretchable. They form an ideal material for ligaments, fascia, tendons, organ capsules and are important components of bone, dentin (teeth), and cartilage.¹
Significance of changes in collagen synthesis
An altered synthesis or degradation of collagen has a clinically important significance. For example, collagen is degraded more during starvation, immobility, rheumatoid arthritis, and weightlessness.¹ Therefore, it can be concluded that collagen degradation can be reduced and the synthesis increased by controlled exercise.³ Later on, we will discuss the effect of exercise on collagen fibers in relation to tendon tissue. During therapy with cortisone, the synthesis of collagen is inhibited.¹
Increased synthesis of collagen physiologically occurs in wound healing (scarring). However, increased collagen synthesis also occurs pathologically, e.g., in liver cirrhosis, pulmonary fibrosis, or atherosclerosis.¹ One approach in the treatment of fluoroquinolone-associated tendon pain is to induce increased collagen synthesis. More on this in the article on treatment options.
There are also diseases with a defective collagen structure, such as brittle bone disease (mutation synthesis type I collagen) or Ehlers-Danlos syndrome.
Anatomical features of the tendon
Anatomically, a tendon has two particularly important structures. The first structure critical to understanding tendon disorders is the junction from tendon to muscle. This transition is also called the myotendinous junction.¹
In the myotendinous junction, the collagen fibers of the tendon intertwine with the fine type III collagen fibrils surrounding the entire muscle fibers.¹
The myotendinous junction is particularly important because it is mostly the weakest point of the muscle-tendon unit.⁵ This is particularly important when it comes to tendon ruptures.
It is very rich in nerve receptors and is subjected to great mechanical stress in transmitting muscle contraction to the tendon.⁵
The second important anatomical structure of the tendon is located at the junction of the tendon and the bone. Fibrocartilage is mostly found there, and the collagen fibers of the tendon radiate into the bone.¹ The tendon’s growth starts from the tendon’s transition to the bone.¹
Tendon sheaths
Tendon sheaths occur where the direction of the tendon’s course is diverted or where they rest directly on a bone.¹ Those circumstances increase the mechanical stress that the tendon must endure, requiring lubrication.⁴
Among others, the long tendons of hands, fingers, feet, and toes have tendon sheaths. This, therefore, reduces the friction of the tendon during movement.
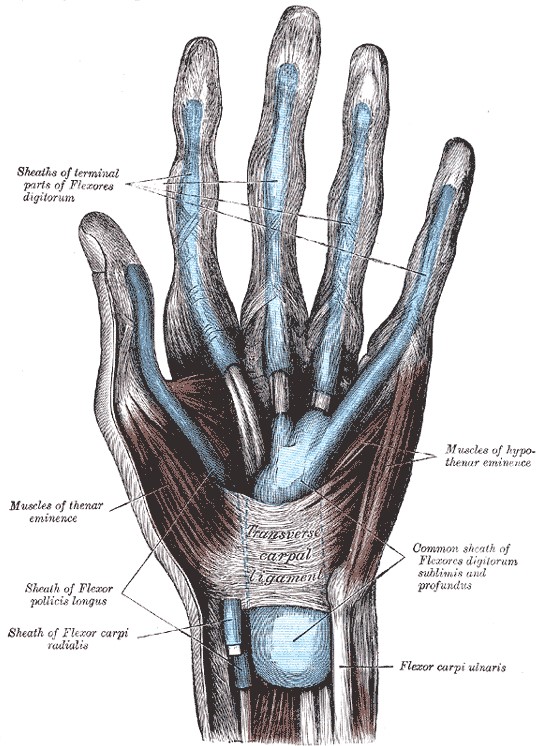
Here we can already build a connection to the clinic, as the difference between a tendon disease, called tendinopathy, and a disease of the surrounding tissue, for example tendinitis, is crucial for prognosis and treatment. The significance of pathological changes in the surrounding tissue is particularly important for the question of how tendon pain arises.
Blood supply to the tendon
When discussing the anatomy of a structure, blood supply, and innervation are always included. Tendons are supplied with blood intrinsically and extrinsically. Intrinsic means that the blood is supplied to the tendon “from the inside.” The intrinsic blood supply of the tendon includes the blood supply from the tendon insertion on the one hand and from the myotendinous junction, which connects the tendon to the muscle on the other.
Extrinsically, meaning originating from the outside, the tendon receives blood from the surrounding tissue. In tendons with a tendon sheath, the blood supply additionally originates from the synovial layer of the tendon sheath.⁴ Depending on the localization and function of the tendon, the blood supply varies. In general, the blood supply of a tendon decreases with higher age and with higher mechanical stress.⁴
Innervation of the tendon
Most nerve fibers do not enter the main body of the tendon but terminate at its surface. However, specialized myelinated nerve fibers occur in the tendon and are mechanoreceptors. Those nerve fibers function as sensors for detecting changes in pressure and tension. This so-called Golgi tendon organ is found most abundantly in the area of the myotendinous junction, i.e., at the transition from tendon to muscle.⁴
Here one can again refer to a widespread therapy option: via these mechanoreceptors, for example, the acupressure technique works to relax the muscles. Suppose one increases the pressure in this area. In that case, a signal of too high tension is transmitted to the central nervous system, which leads to the fact that the musculature is actively relaxed afterward, i.e., with a signal from the central nervous system to the muscle.
Let us return to the innervation of the tendon. In addition to these mechanoreceptors, non-myelinated nerve endings also occur in the tendon. These nerve fibers serve as
nociceptors, i.e., they primarily transmit pain.⁴ Free nerve endings of these nociceptors are represented in the tendon but primarily in the tissue around the tendon.⁵ This again is important for the crucial question of how tendon pain develops. Sympathetic and parasympathetic nerves, i.e., the autonomic nervous system, also occur in tendons.⁴
It can be seen that the anatomy of the tendon is quite complex, and there are many starting points for the development of disease. It is also important to realize that not all tendons can be compared with each other. A tendon with an existing tendon sheath has a different differential diagnosis for existing pain than a tendon without an associated tendon sheath. The same is true for a tendon with high mechanical stress, such as the Achilles tendon, compared to tendons with lower mechanical stress.
In general, however, certain tendencies of tendon tissue can be deduced about disease development, prognosis, and therapy.
In the next article, we take a brief look at the epidemiology of fluoroquinolone-associated tendinopathies, followed by important definitions and classifications of tendinopathies. This additional basic knowledge is crucial for the diagnosis, treatment and prognosis of fluoroquinolone-associated tendinopathy.
Marco Karrer B.Med
Many thanks for their cooperation: Andrea Gall (spelling), Ferdinand Dirsch (SEO, additions, translation into English), Michael Rosar (content, simplifications), Patrick Horisberger (content, simplifications),
First publication: 28.05.2023
Update 15.01.2023
Citation:
- Histologie das Lehrbuch, Ulrich Welsch, Wolfgang Kummer, Thomas Deller Elsevier 5 Auflage 201
- Pathologie das Lehrbuch, Höfler, H. Kreipe, H. Moch 6. Aufl. 2019
- Eccentric exercise training in chronic mid-portion Achilles tendinopathy: a systematic review on different protocols; B Habets, REH van Cingel
- Biology of tendon injury: healing, modeling, and remodeling Sharma and N. Maffuli; 2006
- Pathogenesis of tendinopathies: inflammation or degeneration? Michele Abate et al.

